/ News, Research, Doctorate/PhD, Publication
Is PALB2 a good drug target?
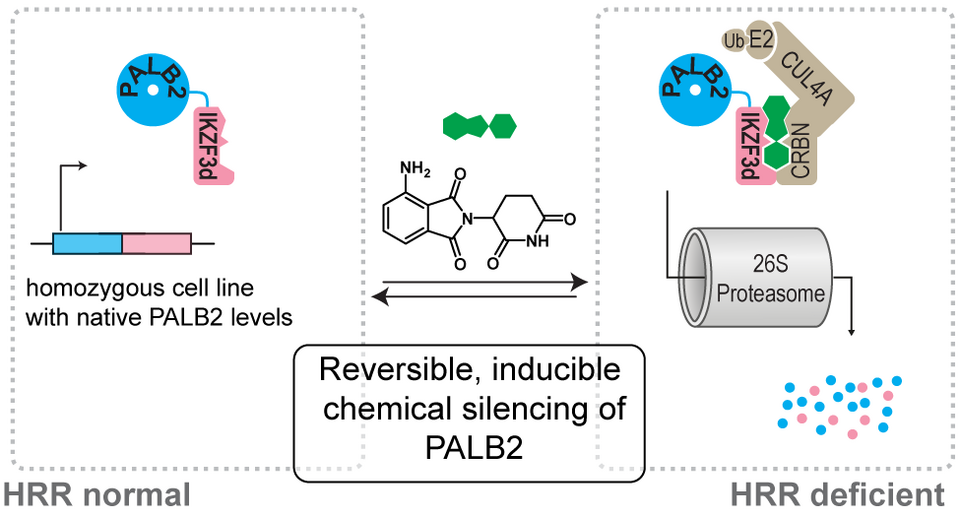
Is PALB2 a good drug target? This is the question that Xinyan Lu tried to answer during his PhD with us by applying many tools from chemical biology to study homologous recombination.
PALB2 is an essential gene involved in homologous recombination (HR). Its name, Partner And Localizer of BRCA2 already hints towards its function: It binds to BRCA2 in nuclear foci during double strand break repairs and interacts with Rad51. Cells that are deficient in BRCA2 are very sensitive to PARP inhibitors, as this knocks out both DNA repair pathways. There was also evidence that certain PALB2 (if silenced with siRNA or impaired by point mutations) can increase sensitivity towards PARB inhibitors - a concept known as synthetic lethality.
Right now, there are no known inhibitors of PALB2. A peptide matching the sequence of the N-termini of BRCA2 has been shown to bind PALB2, but the shorter variant BRCA2(21-39) only binds with a KD of around 0.6 μM. Aramis Keller and Basilius Sauter synthesised different peptides trying to find the shortest binding peptide, but even BRCA2(23-33) still had a length of 11 amino acids and a KD of 8 μM. As peptides have issues passing through cell membranes, it cannot reasonably be used as an inhibitor.
To study what would happen if you could degrade PALB2 by targeted protein degradation, Xinyan Lu created a HEK293T-derived cell line in which he added the IKZF3 degron do its C-Termini. By adding pomalidomide or oder IMiDs, he was then able to degrade PALB2 completely within 6 hours even at moderate concentrations of 1 μM pomalidomide. Then, he studied the homolgous recombination with a plethora of different assays:
- On one assay, a plasmid containing a dysfunctional GFP as well as the sequence required to repair it by homologous recombination was added to the cells. The idea of this assay was that, if GFP gets repaired, HR was working properly. While the cell line with wild type PALB showed no difference in recovery, it the engineered cell line showed the expected decrease in HR efficiency. The amount of cells with repaired GFP was down to 40% compared to DMSO.
- The next assay was a Rad51 foci assay. Cells were stained with antibodies for Rad51 and then treated with camptothecin to trigger DNA double strand breaks (which get repaired by HR). While the number of cells with active Rad51 foci (and thus, active HR) was not changed in the wild type cell line if treated with pomalidomide, the number of foci was significantly lower in the mutated cell line.
Finally, Xinyan Lu checked some compounds that were reported to cause synthetic lethality with PALB2 in siRNA screens. Of these, olaparib—a PARP inhibitor—showed the strongest synthethic lethality, followed by camptothecin.
Although the drugability of PALB is still unanswered, Xinyan Lu has shown that it would be an interesting drug target for patients with deficiencies in PARP or other base excision repair proteins.
