/ News, Research, Publication
Bringing boron containing heterocycles to DNA encoded libraries
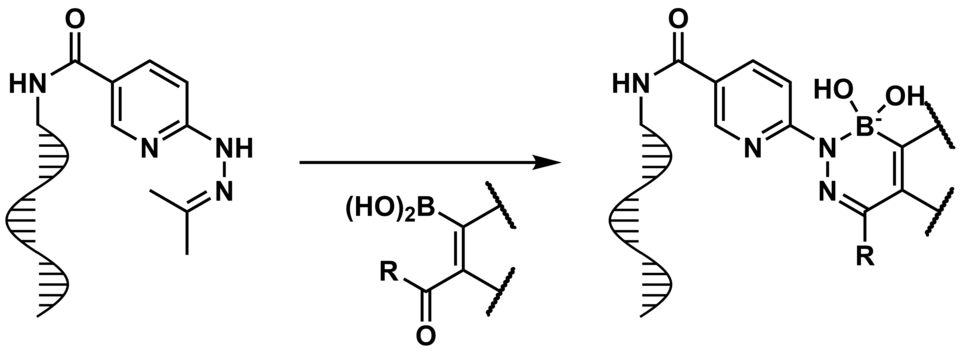
The new paper of Pinwen Cai and Lukas Schneider in Organic Letters shows how an unusual heterocyclic scaffold was made available in DNA encoded libraries. Check it out!
2-Formylphenylboronic acids are known to quickly bind reversibly to hydroxylamines and irreversibly to hydrazines, forming an unusual heterocyclice containing boron and nitrogen (and oxygen in the case of hydroxylamines). Both of these reactions have been shown to work excellent in water (Pascal Schmidt in 2015 in Chemical Science, and Cedrid Stress 2016 in Organic & Biomolecular Chemistry). In the last years, 3 generations of PhD students tried to make this scaffold available to use in DNA encoded libraries - but Pinwen Cai finally found good conditions with general conversion of > 90% (15 examples). The article is available in Organic Letters 2021. The reaction (using protected hydrazines) is not as damaging to DNA as the Suzuki-Miyaura cross-coupling is, and the scaffold could be used to find reversible covalent binders (for example, similar to bortezomib or tavaborole).
"DNA-encoded library (DEL) technology uses DNA tags to track the synthetic history of individual members in a split-and-pool combinatorial synthesis scheme. DEL synthesis hinges on robust methodologies that tolerate combinatorial synthesis schemes while not destroying the information in DNA. We introduce here a DEL-compatible reaction that assembles a boron-containing pyridazine heterocycle. The heterocycle is unique because it can engage in reversible covalent interactions with alcohols─a feature that, until now, has not been deliberately engineered into DELs".
Congratulations, Pinwen and Lukas!
